Electrolyzers and Water: Saving Water, Powering the World with Green Hydrogen
Green hydrogen production isn’t just carbon-free, it can be independent of the grid.
by Dr. Thomas I. Valdez, Principal Engineer for Office of the Chief Technology Officer
Energy created by using water, which does not emit carbon, and is independent of the electrical grid seems far-fetched. This is possible with hydrogen. However, some people question if hydrogen production is an effective use of our water, particularly in drought-stricken parts of the world.
If we want to help the world combat climate change, hydrogen production is an effective use of water. Water is life; as such, making sure communities have access to water for drinking and living is paramount. Water is used by many different industries – to grow food, to produce gasoline, to make steel, and to manufacture other products. The process we use to make hydrogen, called electrolysis, occurs in an electrolyzer, and does use water. However, electrolyzers do not add to our water usage, they displace other technologies which may use more water in the long run.

What is an Electrolyzer and How Does it Work?
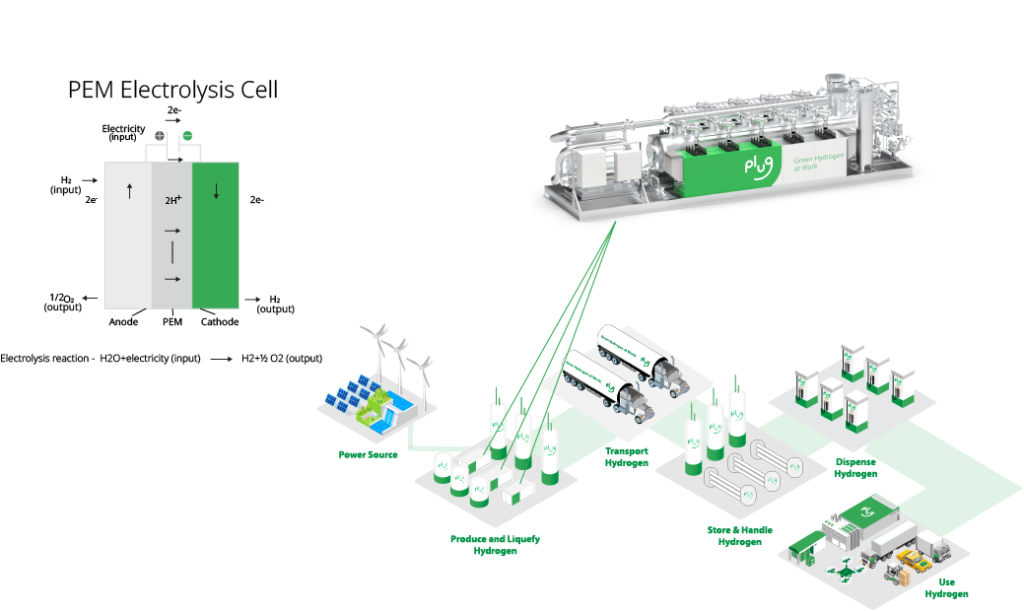
An electrolyzer is an electrochemical machine, which converts water into hydrogen and oxygen gas. Water and electricity are fed to an electrolyzer, and depending on the type of electrolyzer used, water is then converted to protons, hydroxide ions or oxygen ions through electrochemical reactions. As the protons, hydroxide ions, or oxygen ions, complete their respective electrochemical reactions, they liberate hydrogen and oxygen gas from water. Electrolyzers use no chemicals, only water and electricity. When using renewable power, no carbon dioxide or particulate matter is emitted to produce hydrogen and oxygen gas through electrolysis.
As shown in Figure 1, hydrogen is captured, transported, and stored for use by any industry looking to decrease their carbon footprint. The oxygen produced is released into the air. It is projected electrolyzers will soon supply giga-tons of green hydrogen as we transition from fossil to renewable fuels.
Where Does the Water Come From?
The electrolysis process can use different water sources to produce hydrogen. Industrial water, which can include municipal water or groundwater, is the most common source of water used in electrolysis. Different from drinking water, industrial water is used in many different industries including smelting facilities, petroleum refineries, and industries producing food, paper, and chemicals, according to the U.S. Geological Survey.
Prior to being used in the electrolysis process, the industrial water goes through several phases of filtration to ensure maximum purity. Imagine a Brita® water filter you may use at home to clean your tap water. The water filtration process is like a complex Brita® water filter where the water is purified in several stages — removing ions such as calcium, magnesium and other minerals that cannot be used during electrolysis. A schematic for such a water filter is shown in Figure 2.
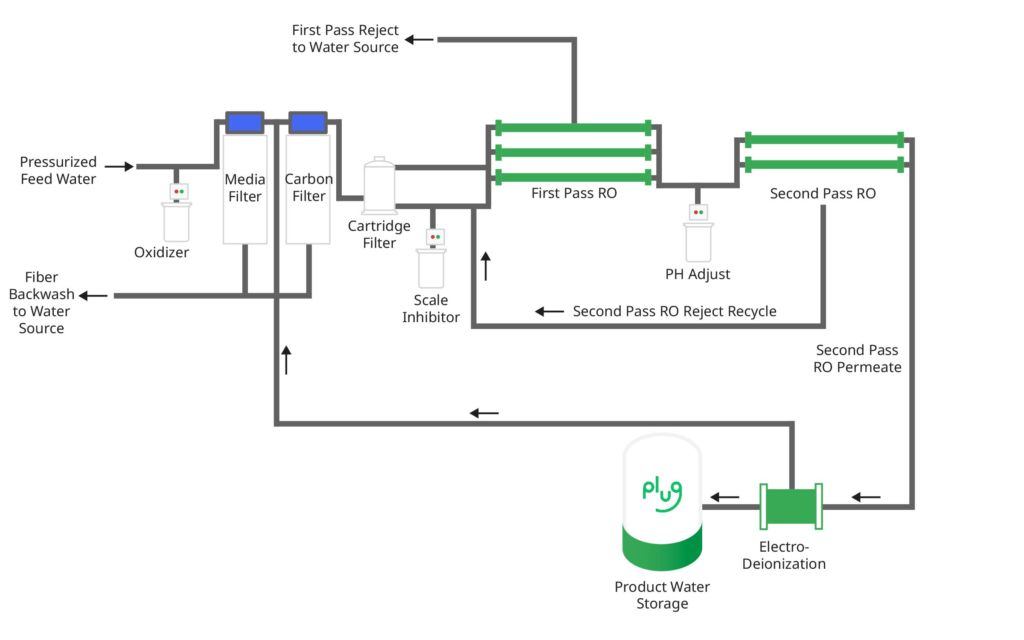
“The water we use to produce hydrogen needs to be as pure as possible,” said Tim Cortes, Chief Technology Officer for Plug. “After hydrogen is produced, any excess water is then recycled to be used for other needs — ensuring the electrolysis process consumes the least amount of water as possible.”
Following the Water
Every nine liters (2.4 gallons) of water contains one kilogram of hydrogen and eight kilograms of oxygen. When you include purifying the water and some water vapor lost with oxygen release, the actual amount is about 12 liters (3 gallons) of water for every kilogram of hydrogen generated through electrolysis.
Water Usage in an Electrolysis Plant
Plug’s STAMP Green Hydrogen Production Site in New York will produce 75,000 kilograms of hydrogen per day. It uses about 908,500 liters (240,000 gallons) of water to produce this amount of hydrogen. On the hottest days of the year, the electrolysis process also uses water for cooling. A hydrogen production site such as STAMP will use an average of 53,000 extra liters (14,000 gallons) of water per day on the hottest days of the year. Thus, the maximum daily water usage at Plug’s STAMP Green Hydrogen Production Site would be 961,500 liters (254,000 gallons). This is about the same amount of water a large dairy farm could use, but less than other agricultural uses such as alfalfa and almond production. Typical industrial water usage is shown in Figure 3.

Figure 3: Typical industrial water usage.
Water Usage in Power Plants
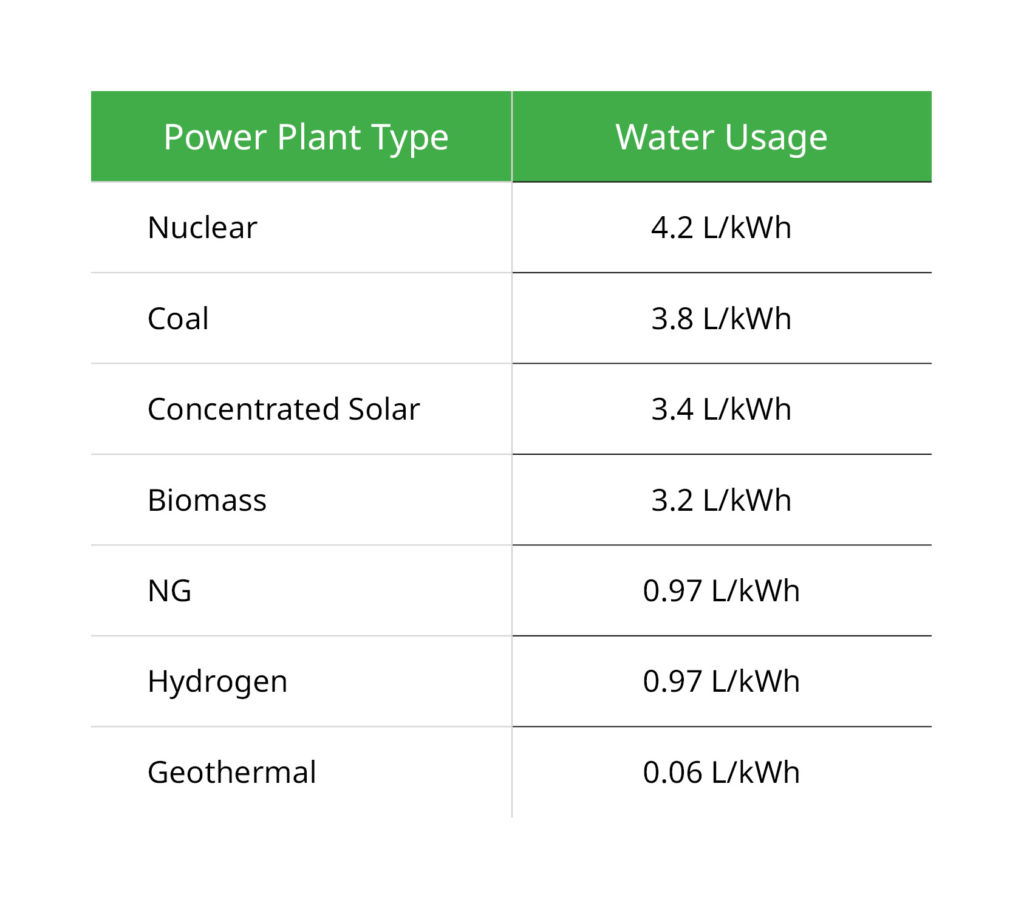
Compared to other power plants, a hydrogen-fed power plant uses less water. A nuclear power plant, for instance, uses 4.2 liters (1.1 gallons) of water for every kilowatt hour of energy produced. Similarly, a coal power plant would use 3.8 liters (1 gallon) of water for every kilowatt hour of energy produced. A hydrogen-fed power plant, however, would use 0.97 liters (0.225 gallons) to provide the same energy, according to the Monarch Partnership, an energy consultancy firm. See Figure 4 below to compare the water usage at various power plants.
Water Usage in Consumer Vehicles
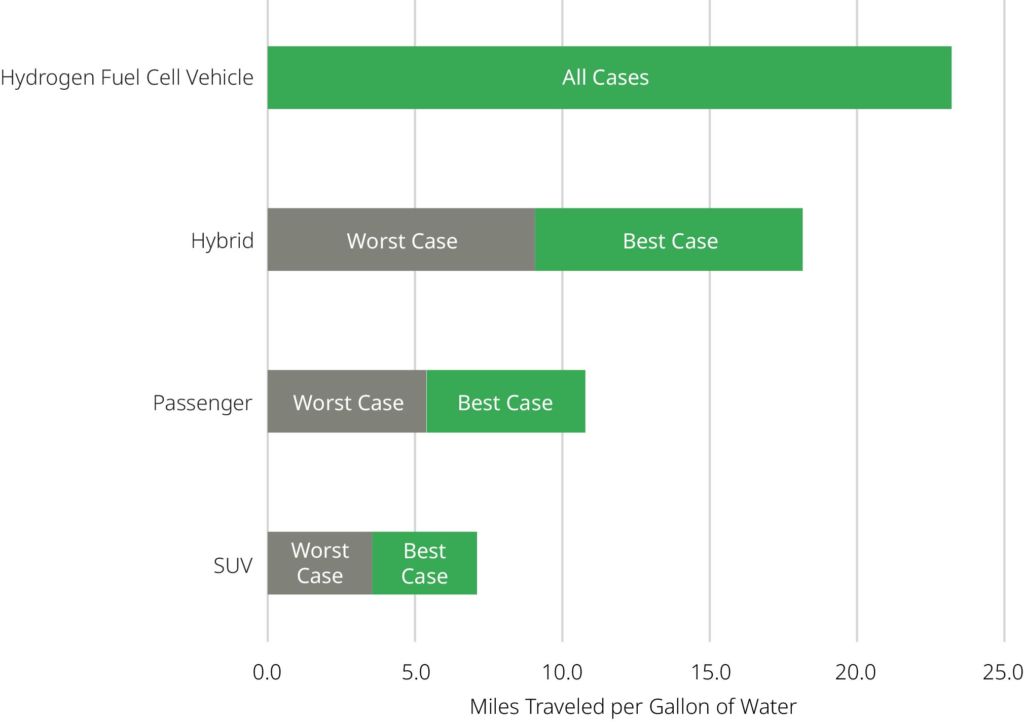
Water is also used by the oil and gas industry to refine petroleum products. To refine three billion liters (800 million gallons) of petroleum products every day in the United States, we use 3.8 to 7.6 billion liters (1 to 2 billion gallons) of water, as estimated by the Lawrence Berkeley National Laboratory. Petroleum products such as gasoline can take up to 22.7 L (6 gallons) of water to produce 3.8 liters (1 gallon) of fuel. Best case, 3.8 liters (1 gallon) of water would be required to produce sufficient gasoline to allow you to drive:
- 7 miles in a typical SUV
- 10.7 miles in a typical passenger vehicle
- 18 miles in the most efficient hybrid vehicles
On the other hand, 3.8 liters (1 gallon) of water could produce sufficient hydrogen for a hydrogen fuel cell vehicle to drive 23.2 miles. This highlights the greater efficiency of fuel cell electric vehicles over traditional gasoline vehicles. Fuel cell electric vehicles can extract more energy from hydrogen than gasoline vehicles can extract from gasoline. Also, it takes less water to make hydrogen than to make gasoline of the equivalent energy. Thus, fuel cell vehicles make better use of water as a resource. The use of water to produce fuel used in consumer vehicles is best highlighted in Figure 5.
Plug CTO Cortes and other experts also agree the upswing in hydrogen availability would mean industries such as steel and cement manufacturers could reduce their carbon footprint.
However, most of the hydrogen used today is produced by a process called steam methane reforming. In steam methane reforming, steam is used to convert natural gas to hydrogen and carbon dioxide. A 2020 study by the German Energy Agency reported steam methane reforming has a cradle-to-grave water consumption of 6 to 13 liters (1.6 to 3.4 gallons) of water for every kilogram of hydrogen generated. This is similar to the water required for electrolysis. However, a steam methane reforming plant would also release approximately 9 kilograms of carbon dioxide for every kilogram of hydrogen generated per the Department of Energy (GREET Model). Thus, Plug’s STAMP Green Hydrogen Production Site could keep over 675,000 kilograms of carbon dioxide from escaping into our atmosphere every day.
“The electrolysis pathway has been proven to be the more environmentally-friendly route to producing hydrogen when using renewable energy,” Cortes said.
Plug’s Commitment
Plug is actively pursuing methods of reducing water consumption in hydrogen production. These include dry cooling methods, higher-efficiency water treatment technologies, and building water treatment facilities like the water treatment facility Plug is building in California. Plug is focused on building an end-to-end green hydrogen ecosystem to help businesses decarbonize their operations, reducing both their carbon and water footprint. Electrolyzers are a critical component of that ecosystem and understanding water usage is key to their benefit.
The post Electrolyzers and Water: Saving Water, Powering the World with Green Hydrogen appeared first on Plug Power.